Introduction
Bacterial resistance to antimicrobials has a genetic basis that can be intrinsic and/or extrinsic to the microorganism. In recent years, it has gained importance due to the increase in in-hospital mortality, largely because of the irrational use of antimicrobials, which has led it to be declared one of the top 10 threats to public health
1
. Additionally, healthcare costs have risen due to the use of expensive antibiotics, longer hospital stays, and a social impact on the wages and productivity of the affected patient
2
.
It is known that the spectrum of antimicrobial resistance is variable, thus microorganisms are classified as multidrug-resistant (MDR) when they show no sensitivity to at least one agent in three or more antimicrobial categories; extensively drug-resistant (XDR) when they show no sensitivity to at least one agent in all categories except one or two, that is, bacterial isolates remain sensitive to only one or two families; and pandrug-resistant (PDR) microorganisms when they show no sensitivity to any agents in all categories of antimicrobials, meaning that no tested agents are effective for that organism
3
.
The microbiological map is a document that provides information about the microorganisms circulating in different hospital departments, evaluates the sensitivity of these microorganisms to the antimicrobials in use, thus contributing to the initiation of an effective and timely empirical treatment in patients with infections, reducing hospital stay, and decreasing healthcare costs
4
.
In some hospitals in Peru, such as in Lima, Arequipa, or Trujillo, microbiological maps have been developed, but in many other regions of the country, this information is lacking. It is important to mention that after the COVID-19 pandemic, the irrational use of antimicrobials for the treatment of SARS-CoV-2 pneumonia significantly contributed to the advance of antimicrobial resistance
5
. Due to the lack of a local microbiological map and the high number of reported infections by multidrug-resistant pathogens, this study aims to identify the microorganisms and their antimicrobial resistance profiles in infections developed in clinical and surgical services.
Methodology
This is a descriptive cross-sectional study that evaluated the results of cultures and antibiograms from clinical samples of patients with suspected infection, who were admitted between January and December 2021 in a hospital located in the Peruvian Jungle. This hospital is one of the main healthcare centers in the Loreto region, with 191 hospital beds, including 56 in the Emergency Department: 42 for adults, 10 for pediatrics, and 4 for obstetrics and gynecology. Additionally, there are 21 beds in the Intensive Care Unit (ICU): 11 for adults, 4 for neonates, and 6 for pediatrics. Other departments include 14 beds in Pediatrics, 26 in Surgery, 19 in Obstetrics and Gynecology, 14 in Traumatology, 29 in Internal Medicine, and 12 in Infectious Diseases. The hospital serves a population of 240,000 people.
For microbiological identification, the VITEK® 2 (bioMérieux) system was used, which is an automated spectrophotometric system for assessing microbial (bacteria and fungi) growth and susceptibility to antimicrobials, according to updated cutoff points from the Clinical and Laboratory Standards Institute (CLSI) guidelines. The type of microorganism, antimicrobial sensitivity, source of isolation, patient's age, and hospital department were identified.
The study was approved by the hospital's Ethics Committee. Data were collected in an Excel spreadsheet (version 2016), coded, tabulated, and analyzed using SPSS Statistics version 25.00. Descriptive statistical analysis was performed, including measures of central tendency and frequency distribution.
Results
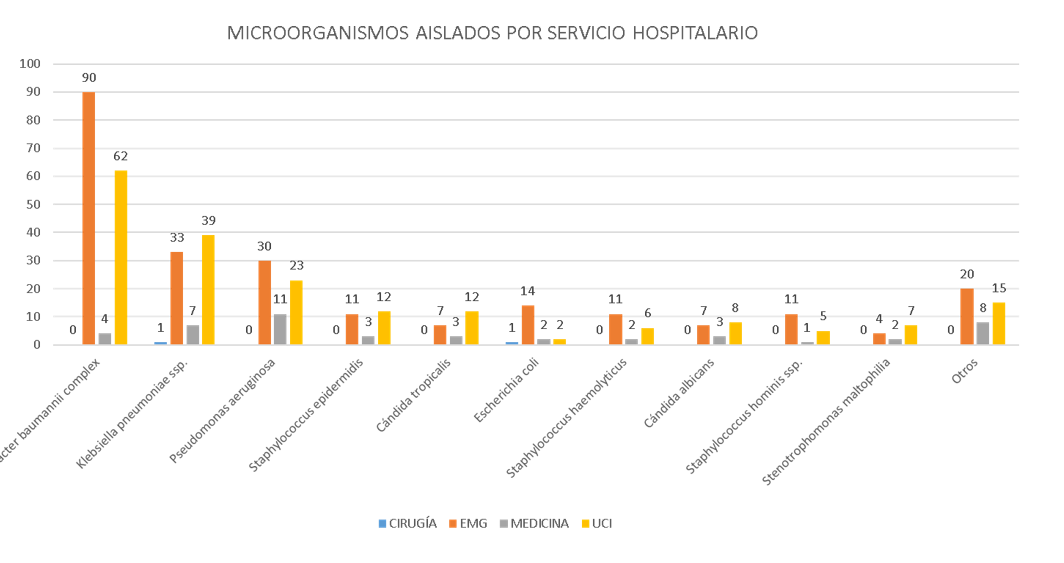
A total of 477 positive cultures were identified from 453 patients; 262 (54.9%) cultures came from bronchial secretion samples, 168 (35.2%) from blood cultures, 31 (6.5%) from urine cultures, and five or fewer from wound secretion, laminar drain, central venous catheter tip, tracheal secretion, cerebrospinal fluid, pleural fluid, and peritoneal secretion samples. The age of the patients ranged from one day old to 88 years (median of 56 years). The microorganisms most regularly isolated were *Acinetobacter baumannii* complex and *Klebsiella pneumoniae* ssp., found most frequently in bronchial secretion cultures from the Emergency Department and Intensive Care Units (Figure 1).
Among the group of Gram-negative bacteria, *Acinetobacter baumannii* complex showed 53% sensitivity to tigecycline and 97% to colistin, while it exhibited 97% resistance to meropenem and 98% to imipenem, being XDR in 88.5% of cases. Regarding *Klebsiella pneumoniae* ssp., 58% were ESBL (+), with 10% sensitivity to ceftazidime and 100% resistance to ceftriaxone, in addition to 44% and 34% resistance to meropenem and imipenem, respectively, being MDR in 56.3% of cases. In the case of *Pseudomonas aeruginosa*, it showed 96% sensitivity to colistin and 70% to amikacin, along with 85% and 82% resistance to meropenem and imipenem, being XDR in 54.7% of cases. As for *Escherichia coli*, 89% were ESBL (+) with 6% sensitivity to ceftazidime and 13% to ceftriaxone (Table 1).
Regarding Gram-positive bacteria, all were isolated from blood cultures; *Staphylococcus epidermidis* was the most frequent, followed by *Staphylococcus haemolyticus*, with 88% and 100% methicillin resistance, respectively, and no resistance to vancomycin was reported for either microorganism. As for *Staphylococcus aureus*, 67% were methicillin-resistant, with no reported vancomycin resistance. Regarding *Enterococcus faecium*, the only isolated strain was resistant to ampicillin but sensitive to vancomycin (Table 2).
In terms of fungi, they were isolated in a total of 43 samples, of which the antifungal susceptibility profile was performed in only six cultures; *Candida parapsilosis* was found in the blood culture of a 66-year-old patient, showing resistance to fluconazole and voriconazole (Table 3).
Figura 1.
Microorganism isolated according to hospital service in a general hospital in the Peruvian jungle 2021
EMG: Emergencias, UCI: Unidad de Cuidados Intensivos Adulto + Unidad de Cuidados Intensivos Neonatales. Otros: Citrobacter freundii, Enterobacter aerogenes, Enterobacter cloacae complex, Morganella morganii ssp., Proteus hauseri, Proteus mirabilis, Providencia stuartii, Pseudomonas stutzeri, Serratia marcescens, Sphingomonas paucimobilis, Enterococcus faecalis, Enterococcus faecium, Kocuria kristinae, Staphylococcus aureus, Staphylococcus capitis, Staphylococcus lugdunensis, Staphylococcus saprophyticus, Staphylococcus warneri, Cándida ciferrii, Cándida krusei, Cándida parapsilosis.
Discussion
In our institution, 74.6% of positive cultures were found to correspond to gram-negative bacteria, followed by gram-positive bacteria at 16.4% and fungi accounting for the remaining 9%. There was a high frequency of bronchial secretion cultures from patients hospitalized in Emergency and Intensive Care Units. Similar findings have been described in microbiological maps of other hospitals in Latin America and our country
6
8
, primarily related to healthcare-associated infections (HAIs). A very high rate of resistance and multidrug resistance was observed in both gram-negative and gram-positive bacteria.
HAIs are more frequent in the respiratory tract, bloodstream, surgical sites, skin-mucosa, and urinary tract. During the SARS-CoV-2 pandemic, there was an increase in HAIs, especially in patients admitted to critical areas, as reported here. Thus, performing antimicrobial cultures was timely to specifically identify associated pathogens
2
,
9
10
.
The pandemic situation enabled greater access to cultures and confirmed the increased antimicrobial resistance profile, similar to reports from a health institution in Colombia
12
. he ICU and Emergency areas reported the highest isolation of microorganisms in the present study, associated with the higher frequency of invasive techniques in patients, which facilitated the entry and subsequent development of infection by these microorganisms. The low frequency of positive cultures in other hospital services is probably due to the lack of resources for sample collection, as critical areas are prioritized, or the lack of awareness among staff about the need to confirm infections (especially HAIs) in non-critical areas of the hospital.
Within the gram-negative bacteria group, Acinetobacter baumannii complex was the most frequently isolated, at 32.7%, with very low sensitivity to imipenem and meropenem, 2% and 3% respectively. This is alarming, as these are reserve drugs typically used empirically for complicated infections, and their low effectiveness is evident in the results shown
13
.
In recent years, Acinetobacter baumannii complex has significantly increased its prevalence, along with its resistance mechanisms
14
15
. A study conducted between 2012 and 2016 on the microbiological profile and antibiotic susceptibility in two highly complex hospitals of Peru's social health insurance system showed E. coli as the main isolated gram-negative organism
16
; in contrast with our study, this highlights the need to continue conducting antimicrobial susceptibility studies to provide an updated overview.
Additionally, 47% resistance to tigecycline was observed; although the first reported appearance of tigecycline resistance was in 2007, a recent study on the prevalence of Acinetobacter baumannii complex showed tigecycline resistance to be below 5.5% in Korea, India, and China (17). Concerns were raised about the utility of this drug for infections associated with Acinetobacter baumannii complex in our region.
The presence of Acinetobacter baumannii complex at such high frequencies suggests that contact isolation measures are inadequate, emphasizing the need for strict adherence to hand hygiene by healthcare personnel as a fundamental part of controlling an endemic situation caused by this pathogen
18
.
Given the high frequency of resistant microorganisms in the study location, such as Acinetobacter baumannii complex, Klebsiella pneumoniae ssp., and Pseudomonas aeruginosa, empirical therapies should include combinations of reserve antimicrobial agents such as colistin, ampicillin/sulbactam, tigecycline, carbapenems, ceftazidime/avibactam, aztreonam, or aminoglycosides. The main focus should be to isolate and properly characterize the microorganism, and, even more importantly, to implement appropriate preventive measures.
Regarding gram-positive bacteria, Staphylococcus aureus had a low frequency compared to other hospitals
19
, likely because few skin lesion isolations were performed in the present study.
Coagulase-negative Staphylococcus (CNS) is often considered the most frequently isolated microorganism in microbiology laboratories
20
. In our study, it accounted for 87% of all gram-positive microorganisms, but the clinical significance of these bacteria could not be determined, as they might have been considered contaminants and/or pathogenic microorganisms. Nonetheless, it is suggested to review protocols for proper blood culture collection and emphasize staff training to avoid confusion in the clinical management of patients.
The prevalence of fungi in blood cultures was low, with greater presence in bronchial secretions. The most frequently isolated fungus was Candida albicans (42.9%), as found in a national hospital
21
, and its resistance rate remains low.
In relation to antimicrobial use, it is crucial to optimize therapy in hospitals. In this regard, it is important to mention that in some institutions in the country, there is still a high frequency of unjustified practices related to these drugs, which contributes to an increase in antimicrobial resistance
22
23
. The use of antimicrobials such as aminopenicillins associated with beta-lactamase inhibitors (BLIs), carbapenems, glycylcyclines (tigecycline), and glycopeptides should be monitored by the Antimicrobial Optimization Committee and the Antimicrobial Optimization Program Unit, hospital entities that help optimize the appropriate use of antimicrobials. These entities promote positive changes in prescribing practices and the use of cost-effective treatments, contributing to better clinical outcomes for patients with infections while reducing the selection of resistant microorganisms and the risks to patients from antimicrobial use. As observed, there is a high rate of resistance in both gram-negative and gram-positive bacteria, necessitating appropriate epidemiological surveillance
24
25
.
This microbiological map is a passive study based on microbiological samples; as such, it is not possible to determine exactly how many and which of the described microorganisms were colonizers and/or contaminants. Moreover, it was not possible to establish which pathogens caused HAIs or the positivity rate of cultures due to the lack of total culture sample data and the genes involved in resistance. However, despite these limitations, the present study constitutes one of the first local reports, and despite including only one hospital, it is important to understand the situation in our region, providing evidence to continue developing the regional microbiological map, which will offer indispensable information for optimizing antimicrobial therapy in the management of infections.
Conclusion
As a conclusion, our study demonstrates the relevance of identifying pathogens and their resistance profiles, particularly in critical areas of hospitals. This allows optimizing antimicrobial use and improving patient outcomes, especially in post-pandemic scenarios with a heightened resistance profile.