Effect of Consumption of Camu Camu Fruit Extract on Sperm DNA Integrity in Mice Pretreated with a Single Dose of Cyclophosphamide
Efecto del Consumo del Extracto del Fruto “Camu Camu” en la Integridad del ADN Espermático de Ratones Pretratados con Dosis Única de Ciclofosfamida | “Camu Camu”果实提取物对单次环磷酰胺预处理小鼠精子DNA完整性的影响
Keywords:
DNA fragmentation, Myrciaria dubia, semen, mouse, Camu-camu, CyclophosphamideAbstract
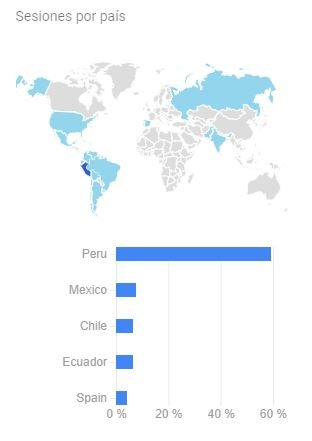
Introduction: Myrciaria dubia known as “camu camu” is a fruit that grows in the Amazon and its main characteristic is its high content of vitamin C. Ascorbic acid has a protective role in spermatogenesis as it is a compound that has excellent reducing action. The purpose of this research was to evaluate in vivo the cytoprotective capacity of the aqueous extract of the fruit of Myrciaria dubia (Kunth) McVaugh “camu-camu” against the mutagenic damage produced by the antineoplastic drug cyclophosphamide (CP) on the male germ line. Methodology: Mice (n= 60) were divided into five treatment groups: T1= negative control (without treatment); T2 ingested the aqueous extract (10mgkg-1), T3 ingested the aqueous extract (50mgkg-1), T4 ingested the aqueous extract (100mgkg-1); T5 is the positive control. All of them were injected with a single dose of CP (50 mgkg-1) intraperitoneally. Treatment with camu-camu continued for 45 days, then the mice were euthanized to determine sperm quality and the frequency of DNA damage using the Index protocol. Sperm DNA fragmentation – Halomax protocol. Results: The effect of camu-camu extract was observed in all trials (p< 0.05) compared to the negative control. Group T4, which was administered the highest concentration of the aqueous extract of the fruit, evidenced the cytoprotective effect of camu-camu (p< 0.05). Conclusion: The damaging effect on DNA due to the oxidative action of CP is inhibited by the aqueous extract of the “camu camu” fruit.
Downloads
References
Roa-Meggo Y. 2012. La infertilidad como Problema de salud Pública en el Perú. Rev peru ginecol obstet 58: 79-85. https://doi.org/:10.31403/rpgo.v58i77
Lourenço M.L., Moura G.A., Rocha Y.M., Rodrigues J.P., Monteiro P.B. 2023. Impact of sperm DNA fragmentation on the clinical outcome of assisted reproduction techniques: a systematic review of the last five years. JBRA Assisted Reproduction 27(2):282–291. https://doi.org/10.5935/1518-0557.20220057
Chowdhury A.R. 1987 Effect of pharmacological agents on male reproduction. Adv Contracept Deliv Syst. 3(4):347-52.
Moskovtsev S.I., Willis J., White J., Mullen B.M. 2009. Sperm DNA Damage: Correlation to Severity of Semen Abnormalities. Urology 74 (4):789-793. ISSN 0090-4295, https://doi.org/10.1016/j.urology.2009.05.043.
Crook T.R., Souhami R.L., McLean A.E. 1986 Cytotoxicity, DNA cross-linking, and single strand breaks induced by activated cyclophosphamide and acrolein in human leukemia cells. Cancer Res. 46: 5029–5034. https://doi.org/10.1007/BF00686152
Hemminki K., Kallama S. (1986). Reactions of nitrogen mustards with DNA. IARC scientific publications, (78): 55–70. PMID: 3583398.
Acosta L, Núñez V, Vásquez J, Pino J, Shiga B. 2012. Dosis única de Ciclofosfamida disminuye la calidad espermática y el epitelio germinal masculino en ratones. Revista Peruana de Biología 19(2):193-198. https://doi.org/10.15381/rpb.v19i2.840
Vigil P, Bustos-Obregón E. 1985. Alkylating agents and mouse spermatogenesis: effects of a single dose of cyclophosphamide. Andrología 17(3): 276-82. https://doi.org/10.1111/j.1439-0272.1985.tb01002.x
Elangovan N., Chiou T.J., Tzeng W.F., Chu S.T. 2006. Cyclophosphamide treatment causes impairment of sperm and its fertilizing ability in mice. Toxicology 222 (1-2): 60-70. https://doi.org/10.1016/j.tox.2006.01.027
Ramos S., Goessler, K.F., Ruiz R.J., Ferrari O., Polito M., Salles M.J. 2012. Exercise protects rat testis from cyclophosphamide-induced damage. Acta Scientiarum. Biological Sciences. 35(1):105-113. https://doi.org/10.4025/actascibiolsci.v35i1.12475
Sancar A., Lindsey-Boltz L.A., Unsal-Kacmaz K., Linn S., 2004. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem; 73: 39 –85. https://doi.org/10.3390/genes5010147
Brinkworth M.H., Nieschlag E. 2000 Association of cyclophosphamide-induced male-mediated, fetal abnormalities with reduced paternal germ-cell apoptosis. Mutat Res 447(2):149-54. https://doi.org/10.1016/S0027-5107(99)00189-X
Collazos C., White P., White H., Viñas E., Alvistur E., Urquieta R., Vásquez, J., Díaz C., Quiroz A., Roca A., Hegsted, M., Brandfield, R.1957. La composición de los alimentos peruanos. Anales De La Facultad De Medicina, 40(1):232–265. https://doi.org/10.15381/anales.v40i1.10737
Fraga C.G., Motchnik P.A., Shigenaga M.K., Helbock H.J., Jacobs R.A., Ames B.N. 1991. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. USA; 88(24): 11003-11006. https://doi.org/10.1073/pnas.88.24.11003.
Niki E. 1991 Vitamin C as an antioxidant. World Rev Nutr Diet.64:1-30. https://doi.org/10.1159/000418567
O’Donoghue J., Barber E.D., Hill T., Aebi J. Fiorica L. 1999. Hydroquinone: Genotoxicity and Prevention of Genotoxicity Following Ingestion. Food Chem. Toxicol 37: 931-936. https://doi.org/10.1016/S0278-6915(99)00084-8
Alvis R. 2010. Detección del efecto antimutagénico del extracto acuoso del fruto de Myrciaria dubia H. B. K. Mc Vaugh “camu camu”, utilizando la prueba in vivo de micronúcleos. Tesis para optar al Título Profesional de Biólogo con mención en Biología Celular y Genética. Escuela Académica Profesional de Ciencias Biológicas. Facultad de Ciencias Biológicas. Universidad Nacional Mayor de San Marcos. 91 pp. https://cybertesis.unmsm.edu.pe/handle/20.500.12672/1357
Alvis R., Pino J., Gonzáles J., Francia J.C., Shiga B. 2010. Efecto citoprotector del camu-camu Myrciaria dubia en tres líneas celulares de ratón expuestos in vivo a bromato de potasio. Revista Peruana de Biología 17(3):389-382. https://doi.org/10.15381/rpb.v17i3.17.
OMS. 2023. https://www.paho.org/es/noticias/4-4-2023-oms-alerta-que-cada-seis-personas-padece-infertilidad. (Revisado el 05/10/23)
National Research Council. 1996. Guide for the Care and Use of Laboratory Animals. National Academy Press. Washington, DC. Disponible en: https://www.ncbi.nlm.nih.gov/books/NBK232589/
Sai K., Umemura T., Takagi A., Hasegawa R., Kurokawa Y. 1992. The protective role of glutathione, cysteine and vitamin C against oxidative DNA damage induced in rat kidney by potassium bromate. Jpn J Cancer Res. 83(1):45-51. https://doi.org/10.1111/j.1349-7006.1992.tb02350.x.
Samarth R.M., Khan T., Srivas S., Mishra P.K., Tiwari, R.R. 2018. Evaluation of cyclophosphamide-induced genotoxicity and cytotoxicity in cultured human lymphocytes. J Radiat Res 9: 28 - 32. https://inis.iaea.org/search/search.aspx?orig_q=RN:50003043
Odagiri Y, Takemoto K, Fenech M. 1994. Micronucleus induction in cytokinesis-blocked mouse bone marrow cells in vitro following in vivo exposure to X-irradiation and cyclophosphamide. Environ Mol Mutagen 24(1):61-7. https://doi.org/10.1002/em.2850240108.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Revista de la Facultad de Medicina Humana

This work is licensed under a Creative Commons Attribution 4.0 International License.